Abstract
Background:
Both standard half-life (SHL) and extended half-life (EHL) factor replacement products are used to treat patients with hemophilia A (hem-A) and B (hem-B). A comparison of international unit (IU) utilization and expenditures ($USD) was made between patients on SHL and EHL factor replacement products to compare utilization and to determine the impact of switching from an SHL to an EHL product.
Methods:
De-identified claims data from both the Truven Health MarketScan® Research (Truven) and Optum® Clinformatics® (Optum) US claims databases included male patients with hem-A (Truven from Aug 2014-Apr 2018 and Optum from Aug 2014-Dec 2017) and hem-B (Truven from Jun 2014-Apr 2018 and Optum from Jul 2014-Dec 2017) who had data for at least 3 months of product dispensation. The SHL and EHL groups were compared. A separate "switch" analysis using the Truven database examined expenditures and IUs before and after switching from nonacog-α (SHL) to FIX-Fc (EHL). Descriptive statistics were used and medians reported for expenditures and IUs to accommodate for the skewness of data distribution.
Results:
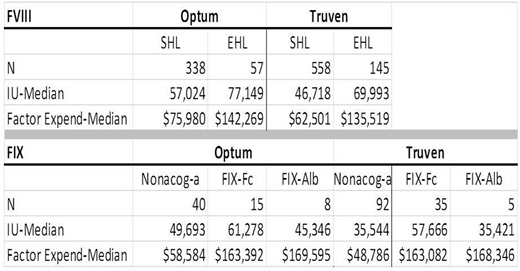
Hem-A: The analysis included 896 SHL and 202 EHL patients, including those who switched from an SHL to an EHL product. Quarterly expenditures and IUs dispensed were analyzed. Both the Optum and Truven databases (see Table) demonstrated higher IU dispensation (Optum: 35%, Truven: 50% higher) and expenditures (Optum: 87%, Truven: 117%) associated with EHL products versus SHL products. In the switch analysis, 35 patients switched from nonacog-α to FIX-Fc; median IUs rose from 61,228 to 75,914 [24%] and median expenditures rose from $78,945 to $155,203 [97%].
Hem-B: The analysis included 50 FIX-Fc, 13 FIX-Alb, and 132 nonacog-α patients. Both databases (see Table) demonstrated higher median expenditures for the EHL products compared with nonacog-α (Optum: 179% for FIX-Fc, 189% for FIX-Alb; Truven: 234% for FIX-Fc, 245% for FIX-Alb). The IU results varied in Optum: FIX-Fc was 23% higher than nonacog-α, but FIX-Alb was 9 % lower than nonacog-α. Similarly, in Truven, the IU results varied: IUs were mixed for the SHL product compared with the EHL products: 62% higher for FIX-Fc than nonacog-α, and approximately the same for FIX-Alb and nonacog-α. In the switch analysis, 16 patients switched from nonacog-α to FIX-Fc; median IUs rose from 62,857 (nonacog-α) to 69,816 (FIX-Fc) [11%], while expenditures rose from $75,064 (nonacog-a) to $210,482 (FIX-Fc) [180%].
Conclusions:
This analysis in more than 1000 patients, unadjusted for severity of disease or treatment scheme, demonstrated in hem-A that higher IU utilization and expenditures were associated with EHL use compared with SHL use, and in hem-B, that higher expenditures were seen with EHL products, while IU dispensation in some cases decreased, remained the same, or increased. In patients switching from the SHL to an EHL product, higher IU dispensation and expenditures were seen. These real-world data may challenge assumptions regarding typical factor usage and expenditures associated with EHL products in patients with hem-A and hem-B. Additional analyses with adjustment for treatment scheme and disease severity are needed.
Tortella:Pfizer Inc.: Employment. Chhabra:Pfizer Inc.: Employment. Alvir:Pfizer Inc.: Employment. Rubinstein:Pfizer Inc.: Employment. Young:Pfizer Ltd.: Employment. Fogarty:Pfizer Inc.: Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal